
Bowman Lab
Department of Biochemistry
Jacobs School of Medicine & Biomedical Sciences
University at Buffalo
Metalloprotein Structure & Function
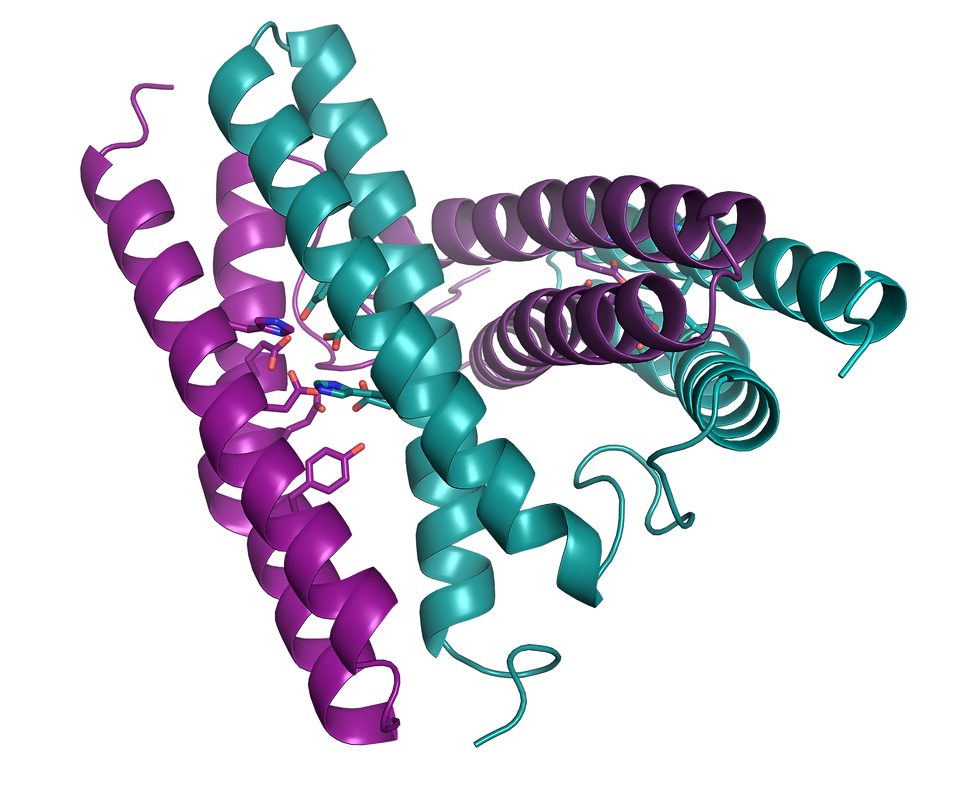
Cartoon ribbon of rubrerythrin, a di-metal protein

Active site of apo rubrerythrin

SeRbr protein crystal in a nylon loop, color bleached out due to change in oxidation state

Cartoon ribbon of rubrerythrin, a di-metal protein
One focus area in the Bowman Lab is focused on pathogenic microorganisms and how they use metals in virulence and pathogenicity. Metals are involved in many reactions that define life: photosynthesis, metabolism, oxygen shuttling, electron transfer, and more. Metals in proteins enable a range of chemical functionality due to different redox states, electronic structures, and coordination environments, empowering an expansive breadth of biomolecular reactivity. In structural research, X-ray and electron sources used in diffraction and CryoEM studies can cause radiation damage, which is often exacerbated in metalloproteins. Additionally, changes in metal oxidation state can occur with standard structural methods.
In the Bowman Lab, we are working to address this gap in available methods for studying metal-containing chemical biology systems with structural techniques by developing ways to couple spectroscopic tools for probing metal centers to structural tools for structure determination.
Technology Development for Ultrasmall Crystals
Recent hardware and software innovations have opened doors to using new cutting-edge crystal-based methods, including serial femtosecond crystallography (SFX), serial synchrotron crystallography (SSX), and three-dimensional electron diffraction (3DED). All of these methods require ultrasmall, well-ordered protein crystals.
The Bowman Group is working to develop better ways to detect, characterize, and handle these ultrasmall crystals.

Schematic with different detection methods for protein crystals
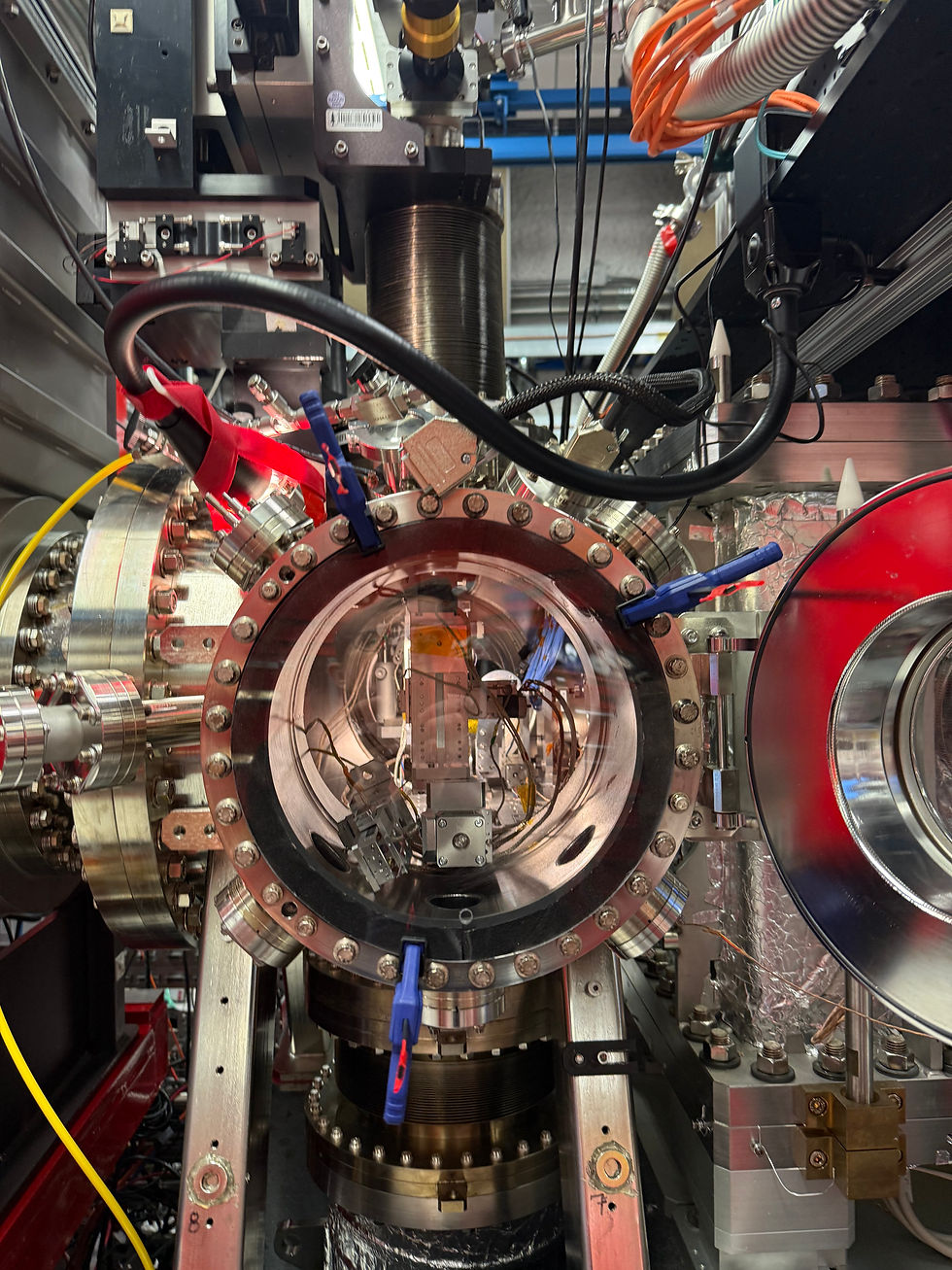
CXI instrument at LCLS

Protein macrocrystal

Schematic with different detection methods for protein crystals
Structural Biology in the Time of COVID-19

Structures of proteins from the SARS-CoV-2 virus

Comparison of X-ray and EM techniques usage during pandemic

Structures of proteins from the SARS-CoV-2 virus
The central role played by structural biology resources was highlighted during the COVID-19 pandemic. Determining structures of key viral proteins fueled development of both vaccines and therapeutics, and helped unravel details of how the virus infects hosts and spreads. The NCC supported many research projects during the pandemic.
The pandemic provided a unique opportunity to investigate the role of structural methods in real-time, since so many of us pivoted so completely to address the needs during the pandemic. We published a perspective piece on the role of structural biology in the time of COVID-19. What we learned is that multiple techniques were necessary to structurally characterize the full range of proteins from this novel virus. The work we and others did during the pandemic underscore the importance of structural biology and the role structural information plays in tackling major societal challenges.